Lynn L. Bergeson, Charles M. Auer, and Richard E. Engler, Ph.D., Present during Bloomberg BNA Webinar “Reviewing New Chemicals Under Amended TSCA: Impact on Innovation”
On June 12, 2017, Bloomberg BNA presented the webinar “Reviewing New Chemicals Under Amended TSCA: Impact on Innovation,” featuring Charles M. Auer, Senior Regulatory and Policy Advisor with The Acta Group (Acta®); Richard E. Engler, Ph.D., Senior Chemist with Acta; and Lynn L. Bergeson, President of Acta; as well as Jeffery Morris, Director, U.S. Environmental Protection Agency (EPA), Office of Pollution Prevention and Toxics; Beth Bosley, President, Boron Specialties LLC; and Robert Mott, Manager, Global Regulatory, Sun Chemical Corporation. After an introduction from Jeff Morris, Charles Auer and Richard Engler kicked off the program with “Old versus New Toxic Substance Control Act Section 5: Overview of the Requirements, EPA’s Outcomes, and Submitters’ Concerns,” where they addressed concerns that have arisen as a result of EPA’s new requirement that requires an EPA determination on all new chemicals. The three alternative determinations at Section 5(a)(3) are:
(A) New chemical presents an unreasonable risk
(B)(i) Available information is insufficient to permit reasoned evaluation of health and environmental effects or (ii)(I) new chemical may present unreasonable risk or (ii)(II) it has substantial production and exposure, or
(C) New chemical not likely to present unreasonable risk
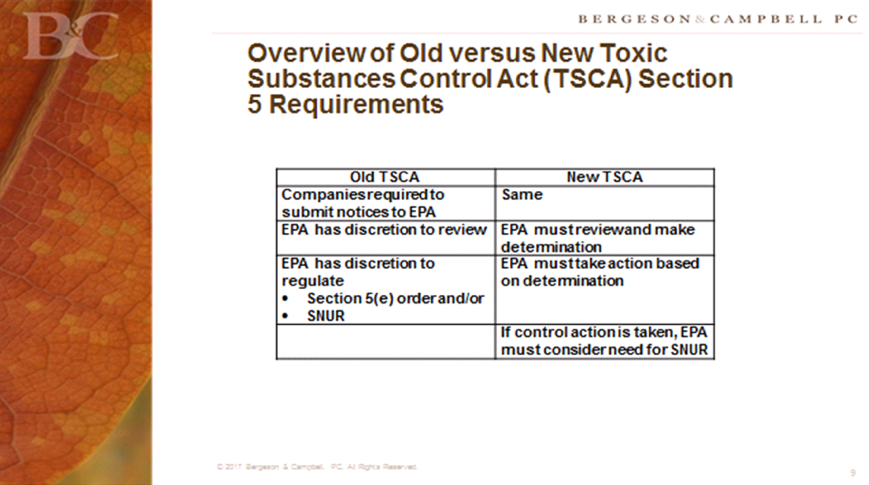
Lynn L. Bergeson’s presentation, “Advice to Chemical Manufacturers,” laid out key points to consider when submitting a premanufacture notice (PNM) for risk assessment. Key slides from Ms. Bergeson’s presentation include:


Acta affiliate Bergeson & Campbell P.C. (B&C®) was a proud sponsor of this webinar. For more information or for resources from the webinar, contact Heidi Lewis at hlewis@lawbc.com.
